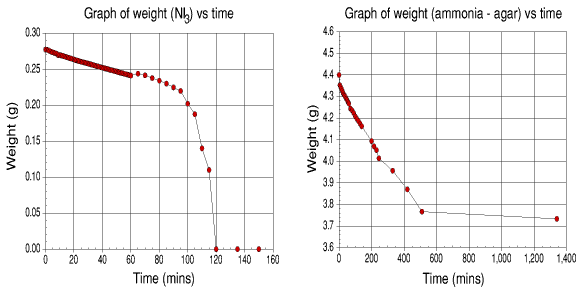
Graphs of weight loss of samples vs. time
Rate of NH3 loss for the ammonia - agar and the sample. Method 1.9
The samples (either NI3 adduct by itself or ammonia - agar gel without NI3) were left on a balance connected to a BBC Micro B computer. The computer was set to take readings from the balance every minute for the first hour, every 5 for the second hour, every 15 for the third hour and then every half hour for a maximum of 24 hours. The point at which there was no considerable amount of weight loss, would be the point at which the ammonia had fully evaporated from the sample.
1. Times for weight loss
The times for the weight losses of for the ammonia - agar then NI3 are set out below.
| Time (mins) | Weight (g) inc. holder | Difference | Weight of sample |
| 0 | 11.7860 | 0.0000 | 4.3997 |
| 5 | 11.7384 | 0.0476 | 4.3521 |
| 10 | 11.7298 | 0.0562 | 4.3435 |
| 15 | 11.7213 | 0.0647 | 4.3350 |
| 20 | 11.7115 | 0.0745 | 4.3252 |
| 25 | 11.7015 | 0.0845 | 4.3152 |
| 30 | 11.6949 | 0.0911 | 4.3086 |
| 35 | 11.6892 | 0.0968 | 4.3029 |
| 40 | 11.6821 | 0.1039 | 4.2958 |
| 45 | 11.6755 | 0.1105 | 4.2892 |
| 50 | 11.6688 | 0.1172 | 4.2825 |
| 55 | 11.6612 | 0.1248 | 4.2749 |
| 60 | 11.6537 | 0.1323 | 4.2674 |
| 70 | 11.6302 | 0.1558 | 4.2439 |
| 80 | 11.6223 | 0.1637 | 4.2360 |
| 90 | 11.6093 | 0.1767 | 4.2230 |
| 100 | 11.5937 | 0.1923 | 4.2074 |
| 110 | 11.5817 | 0.2043 | 4.1954 |
| 120 | 11.5725 | 0.2135 | 4.1862 |
| 130 | 11.5602 | 0.2257 | 4.1740 |
| 140 | 11.5480 | 0.2380 | 4.1617 |
| 200 | 11.4798 | 0.3062 | 4.0935 |
| 215 | 11.4551 | 0.3309 | 4.0688 |
| 230 | 11.4380 | 0.3480 | 4.0517 |
| 245 | 11.3999 | 0.3861 | 4.0136 |
| 330 | 11.3427 | 0.4433 | 3.9564 |
| 420 | 11.2562 | 0.5298 | 3.8699 |
| 510 | 11.1532 | 0.6328 | 3.7669 |
| 1340 | 11.1199 | 0.6661 | 3.7336 |
Summary table for weight loss of ammonia from ammonia - agar sample.
| Time | Weight (g) | Loss (g) | Time | Weight (g) | Loss (g) | Time | Weight (g) | Loss (g) | ||
| 0 | 0.2773 | 0.0000 | 30 | 0.2577 | 0.0006 | 65 | 0.2437 | 0.0026 | ||
| 1 | 0.2770 | 0.0003 | 31 | 0.2572 | 0.0005 | 70 | 0.2415 | 0.0022 | ||
| 2 | 0.2761 | 0.0009 | 32 | 0.2566 | 0.0006 | 75 | 0.2377 | 0.0038 | ||
| 3 | 0.2752 | 0.0009 | 33 | 0.2560 | 0.0006 | 80 | 0.2341 | 0.0036 | ||
| 4 | 0.2743 | 0.0009 | 34 | 0.2554 | 0.0006 | 85 | 0.2300 | 0.0041 | ||
| 5 | 0.2736 | 0.0007 | 35 | 0.2548 | 0.0006 | 90 | 0.2247 | 0.0053 | ||
| 6 | 0.2728 | 0.0008 | 36 | 0.2542 | 0.0006 | 95 | 0.2198 | 0.0049 | ||
| 7 | 0.2720 | 0.0008 | 37 | 0.2538 | 0.0004 | 100 | 0.2020 | 0.0178 | ||
| 8 | 0.2713 | 0.0007 | 38 | 0.2531 | 0.0007 | 105 | 0.1875 | 0.0145 | ||
| 9 | 0.2694 | 0.0019 | 39 | 0.2527 | 0.0004 | 110 | 0.1401 | 0.0474 | ||
| 10 | 0.2698 | -0.0004 | 40 | 0.2521 | 0.0006 | 115 | 0.1100 | 0.0301 | ||
| 11 | 0.2691 | 0.0007 | 41 | 0.2516 | 0.0005 | 120 | 0.0000 | 0.1100 | ||
| 12 | 0.2686 | 0.0005 | 42 | 0.2510 | 0.0006 | 135 | 0.0000 | 0.0000 | ||
| 13 | 0.2679 | 0.0007 | 43 | 0.2505 | 0.0005 | 150 | 0.0000 | 0.0000 | ||
| 14 | 0.2673 | 0.0006 | 44 | 0.2500 | 0.0005 | |||||
| 15 | 0.2666 | 0.0007 | 45 | 0.2493 | 0.0007 | |||||
| 16 | 0.2659 | 0.0007 | 46 | 0.2488 | 0.0005 | |||||
| 17 | 0.2653 | 0.0006 | 47 | 0.2484 | 0.0004 | |||||
| 18 | 0.2647 | 0.0006 | 48 | 0.2477 | 0.0007 | |||||
| 19 | 0.2641 | 0.0006 | 49 | 0.2472 | 0.0005 | |||||
| 20 | 0.2635 | 0.0006 | 50 | 0.2468 | 0.0004 | |||||
| 21 | 0.2627 | 0.0008 | 51 | 0.2460 | 0.0008 | |||||
| 22 | 0.2623 | 0.0004 | 52 | 0.2455 | 0.0005 | |||||
| 23 | 0.2616 | 0.0007 | 53 | 0.2450 | 0.0005 | |||||
| 24 | 0.2611 | 0.0005 | 54 | 0.2444 | 0.0006 | |||||
| 25 | 0.2605 | 0.0006 | 55 | 0.2439 | 0.0005 | |||||
| 26 | 0.2599 | 0.0006 | 56 | 0.2433 | 0.0006 | |||||
| 27 | 0.2593 | 0.0006 | 57 | 0.2428 | 0.0005 | |||||
| 28 | 0.2588 | 0.0005 | 58 | 0.2423 | 0.0005 | |||||
| 29 | 0.2583 | 0.0005 | 59 | 0.2417 | 0.0006 | |||||
| 30 | 0.2577 | -0.0006 | 60 | 0.2411 | 0.0006 |
Summary table for the evolution of ammonia from a sample of NI3. All times in minutes.

Graphs of weight loss of samples vs. time
It can be seen from the graphs that the major loss of ammonia for the agar occurs around 500 minutes. After this point, the evolution of ammonia is greatly reduced.
The NI3.NH3 lost ammonia at a steady rate for the first hour. After this point, the evolution increased until detonation occurred at the two hour mark. This experiment was repeated again and gave the same results.