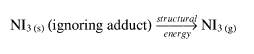
Conclusion
The set of experiments carried out have led to a number of interesting findings. The three most exceptional ones are the delta Hdet (NI3), the number of ammonia molecules in the adduct and the fact that agar can be used as a stabilising agent for NI3.
From the data obtained by both types of bomb calorimetry, the theory regarding bond energy can be seen not to be working. The literature value9 for †Hf (NI3) is 287 ± 23 kJ mol-1 and for the monoammine adduct is 146.6 ± 6 kJ mol-1. Yet the values for the detonation show a delta Hdet of » 20000 kJ mol-1. The amount of energy released on detonation is far greater than that required to form the NI3. Where is this extra amount of energy coming from? Further work is needed here.
The large energy release on detonation could be accounted for as follows. In normal practise for normal compounds, we would vaporise the solid (this would require an input of energy) :
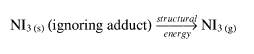
The NI3 is next dissociated. A further input of energy would be required.
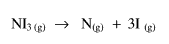
The atoms produced would combine to give N2 and I2. An output of energy would appear at this stage.
Inspection of bond energy tables show that normal bond energies are only of the order of hundreds of kiloJoules.
It is suggested that the source of the detonation energy is structural and that NI3 (s) is in a a structurally metastable state (rather like that of a supercooled liquid), ready at the slightest chance to change to some other structure, with a considerable release of energy. This is supported by the sensitivity of the dry unstablised solid. It explains the role of the stabilisers such as water and agar. The stabilisers act as scaffolding, holding together the metastable crystal structure of NI3. The supporting structure could be the localised structure in the water. The bonding could be the hydrogen bonding in water.
Once the structure and its associated bonding have been removed, the NI3 becomes unstable, its own structure being maintained by only a very few low energy barriers. Detonation (with enough force to include molecular disintegration) follow.
The stabilisation of NI3 also poses a problem, namely that of a gel being able to increase the energy barrier (acting as a negative catalyst) and so stabilising a molecule.
Early experiments performed in this project pointed to agar being able to gel when 0.880 S.G. ammonia was used. If the amount of agar used was kept constant (at around 3.2 g), and the ammonia allowed to boil with constant stirring, the dry gel powder would form the emulsion which will set in time to form the gel. The gelling time for ammonical gels remains around the same for up to 80%. After this time, the time required for the gel increases rapidly. Using just 0.880 S.G. ammonia took eight hours to form the gel. Yet dry NI3 would self detonate after about 7 minutes (this is from the adduct to NI3 to detonation).
NI3 will form when iodine comes into contact with NH3 in the gas or liquid phases. By making the NI3 in situ with the ammonia - agar meant that the NI3 would always be surrounded by a large amount of ammonia. The conjecture was that as the ammonia - agar gel dried, the gel would be formed by the loss of NH3 with a large degree of weak cross links (they would be weak as agar gels will dry out and form either plastics or revert to the powder depending on the amount of time left). By placing the NI3 into the gel at around 40°C, the cross links would act as an inhibitor to vibrations.
The gel would not be able to support the NI3 indefinitely due to the break down of the gel over time. It was able to keep the NI3 stable for up to 24 hours and able to travel (the gels had been prepared at the University of Salford and transported to Liverpool John Moores University to test using the bomb calorimeter - a distance of around 40 miles). No detonation had occurred during this time, the weight of the sample before the transportation and after the transportation being the same.
No stability testing was performed for longer than 24 hours.
The number of moles of NH3 in the adduct has always been a bone of contention with evidence to support both the monoammine and triammine structures1. The present results a direct result of 3 (giving the RMM for the adduct as 446). Observation of this structure could be possible using low energy XRD techniques at low temperatures (ambient temperature of » -35°C). It may also be possible to study this using a low temperature, high speed i.r. For the present experiments, the average scan time was in excess of 4 minutes requisition in the ammonia - agar gel. To be more sure of a value, a scan speed of less than 30 seconds would be desirable and not in the gel.
The adduct could also be determined using solid state nmr, or, by utilising the lone pair on the nitrogen, esr. For the nmr, the NI3 would have to be made up using 14NH3, dried and then placed into 15NH3. The integration of the 15N would show the relative proportions of NH3 to NI3. The major problem with using either nmr or esr is the intrinsic instability of NI3.
In conclusion, this project has now produced more data into this very understudied compound. The values for the enthalpy of detonation (using the glass calorimeter) are all within a small range of each other (giving a confidence regarding the result). The main purpose of the project was to study a very unstable compound and gain some insight into the structure. To this end, the purpose has been achieved with a degree of success.